Oxidation Number
Oxidation Number: Overview
This topic covers concepts such as Oxidation Number, Stock Notation for Oxidation Number, Rules for Calculating Oxidation Number, Oxidation Number in Native State, Oxidation Number of H-atom in compounds, Oxidation Number of O-atom in Compounds, etc.
Important Questions on Oxidation Number
The equivalent weight of in the following reactions would be:
The oxidation number of elements decreases across the period.
Discuss the trends of oxidation number across the period in the periodic table.
Give an example of limitation of oxidation number concept.
Write the limitation of oxidation number concept.
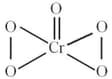
has a structure as shown below:
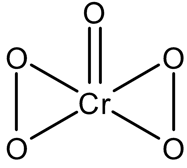
The oxidation number of chromium in the above compound is
has structure as shown

Which of the following oxidation states is/are shown by hydrogen?
The oxidation state of chromium in the final product formed by the reaction between and acidified potassium dichromate solution is
mole of (atomic weight is ) is oxidized to . Calculate the equivalent weight of ferrous ion
One mole of hydrazine loses 10 moles of electrons in a reaction to form a new compound Assuming that all the nitrogen atoms in hydrazine appear in the new compound, what is the oxidation state of nitrogen in ? (Note There is no change in the oxidation state of hydrogen in the reaction)
Which of the following oxidation states is/are shown by hydrogen?
What is the oxidation number of copper in brass?
The oxidation state of nitrogen in dinitrogen trioxide is:
The heating of produces another chromium compound along with gas. The change of the oxidation state of in the reaction is
The formal oxidation numbers of and in the ions and , respectively are
How many of the following species have formal oxidation state of or as
The species with an atom in oxidation state is